Esko recently outlined details of a comprehensive strategy for helping companies adapt their packaging in the face of new regulatory compliance regulations on nutritional labeling. “We are seeing a wave of new legislation being introduced as governments all over the world seek to educate consumers via product packaging,” said Philippe Adam, Vice President of Global Marketing at Esko. “For the food and beverage industry, as well as many pharmaceutical and nutraceutical companies, this means keeping on top of all the various regulations and adapting their packaging and business processes accordingly. With our new tailored solutions and smart software capabilities, we can help.”
The announcement from Esko follows the US Food and Drug Administration’s (FDA) introduction of a modernized Nutrition Facts label for packaged foods, with compliance required by July 2018. The U.S. Department of Agriculture’s (USDA) has since proposed updated nutritional information for meat and poultry products. Regulators in other countries are taking a similar approach, with new guidelines on communicating nutritional information on packaging announced in Canada and France, with others set to follow.
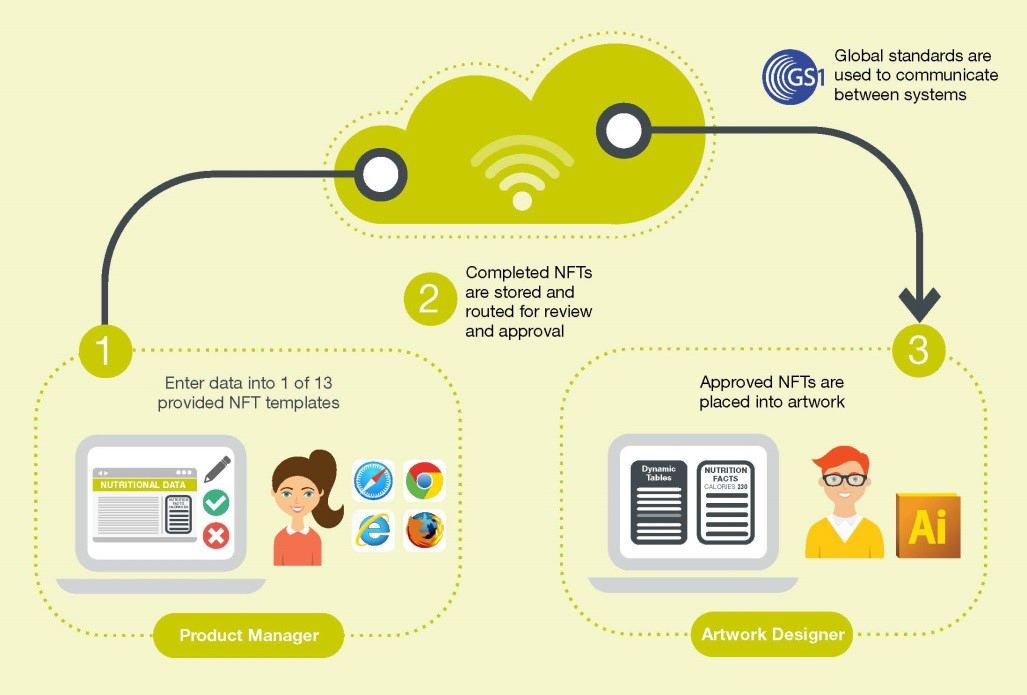
Depending on the needs of each individual organization and where the target data is stored, Esko has devised three clearly defined solutions. Solution 1 starts with the brand owner, Solution 2 starts with the artwork and Solution 3 is full system integration. Each organization can choose the solution most suited to its current situation and be confident that they will optimize their nutritional labeling process and create a single source of truth. Detailed information on the different solution can be found in Esko’s white paper on regulatory compliance process and technology.
In addition, Esko is releasing a feature that pulls nutritional fact data from existing packaging files (AI or PDF files) and converts this to a data stream that can be used on any other piece of artwork to automate artwork creation. This capability is expected to result in timesavings of up to 70% for customers who have to convert all their tables to comply with the FDA regulations.
“It is important that companies can stay ahead of all new regulations and feel confident every single SKU they produce will have compliant packaging,” said Adam. “Esko will continue to support with new templates into which centralized nutrition and ingredient information can be automatically placed. With our solutions in place, new regulations can just be an everyday standard part of doing business and not an exceptional undertaking and disruption.”